BEEASY CONSULTING SRLS
VIALE F. STROZZI, 30 - 50129 FIRENZE (ITALY) * VAT n.: IT07244890484 * REA REGISTRATION n.: FI–689983 * FULLY PAID COMPANY CAPITAL: € 5.000,00

NAVIGATING REGULATIONS, ACCELERATING APPROVALS,
ADVANCING HEALTHCARE
"To see things in the seed, that is genius."
Lao Tzu

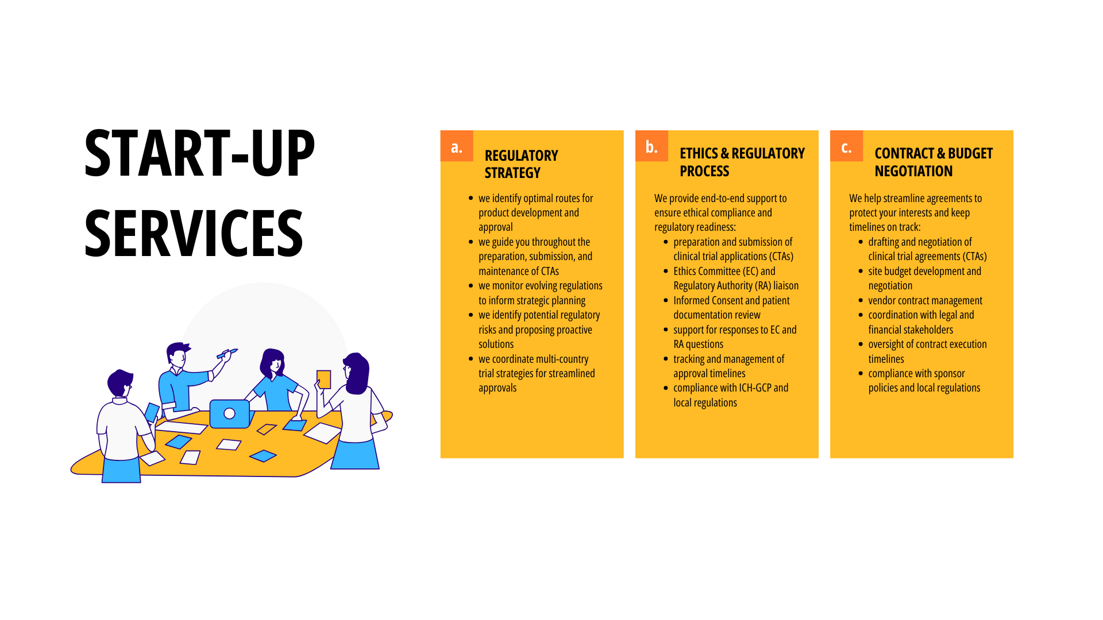
THE KEY ROLE OF START-UP
Randomized controlled clinical studies represent the gold standard for evaluating the safety and efficacy of potential medications and therapies.
However, initiating these studies can be highly complex, particularly when they involve multinational sites governed by diverse laws, regulatory frameworks, infrastructure limitations, and varying standards of care.
Before recruitment can begin, investigational sites must undergo qualification, obtain regulatory approvals, negotiate and finalize clinical study agreements, and complete necessary training while securing clinical supplies, among other critical study-level tasks. Delays in start-up can extend overall study timelines, leading to significant additional costs and potentially jeopardizing study feasibility.
The start-up phase is a critical factor in a clinical study’s success, with activation time often inversely affecting enrollment rates.
STRENGTHEN YOUR REGULATORY STRATEGY
Regulatory delays often prolong clinical study timelines, but a proactive approach can help mitigate delays and improve efficiency, helping your study move forward without unnecessary delays. There isn’t a single predefined solution, but here are some potential options to consider for your clinical study:
- Understand regional regulatory landscapes: mapping out submission timelines and documentation needs early can prevent last-minute surprises.
- Engage early with Regulatory Authorities: request pre-IND meetings or Scientific Advice sessions to clarify requirements and refine your study design with expert feedback.
- Keep connected with Regulatory Authorities throughout the process: beyond early meetings, maintaining ongoing communication with agencies can help address concerns before they become obstacles.
- Implement Adaptive Study designs: using flexible protocols that allow for modifications based on interim data can reduce the need for lengthy amendments and reapprovals.
- Strengthen Chemistry, Manufacturing, and Controls (CMC) processes: ensuring consistency in manufacturing and analytical methods can prevent regulatory setbacks related to product quality.
- Prepare high-quality documentation: informed consent forms, investigator brochures, and study protocols must be clear, accurate, and compliant with regulatory standards to avoid unnecessary revisions.
- Leverage Accelerated Approval pathways: utilize options like Fast Track, Breakthrough Therapy, or Priority Review to expedite regulatory review.
- Optimize Submission Packages: ensure completeness, accuracy, and consistency in regulatory submissions. Electronic filing can streamline the process and reduce review times.

EXPERIENCE IN ACTION
See the data that sets us apart.


INTERESTED IN OUR SERVICES? LET’S TALK.
CONNECT
WITH BEEASY
Contact us here or check us out on our LinkedIn.
Get in touch today and discover how we can support your next steps.

